Introduction
The automotive industry has been undergoing a significant transformation in recent years, with a growing emphasis on sustainability and reducing carbon emissions. Among the various alternative propulsion technologies being explored, hydrogen fuel cell technology has emerged as a promising solution. Hydrogen-powered vehicles offer the potential for zero-emission transportation while addressing some of the limitations of battery electric vehicles (BEVs). In this article, we will explore the principles of fuel cell technology, its advantages, challenges, and the current state of hydrogen-powered vehicles.
The automotive industry has been undergoing a significant transformation in recent years, with a growing emphasis on sustainability and reducing carbon emissions. This shift is being driven by increasing environmental awareness, stricter emissions regulations, and the need to mitigate climate change. As a result, automakers are actively exploring alternative propulsion technologies to usher in a cleaner and greener era of transportation.
One of the most promising solutions on the horizon is hydrogen fuel cell technology. Hydrogen-powered vehicles represent a leap forward in eco-friendly transportation and offer several distinct advantages over their counterparts. While battery electric vehicles (BEVs) have gained considerable attention and market share, hydrogen fuel cell vehicles provide a compelling alternative that addresses some of the limitations associated with traditional electric vehicles.
Hydrogen fuel cell vehicles operate on a simple yet ingenious principle: they generate electricity on demand through an electrochemical process, wherein hydrogen gas is combined with oxygen from the air to produce electricity, water vapor, and heat. This process takes place in a fuel cell stack, and the electricity generated powers an electric motor to drive the vehicle. Unlike combustion engines, this process produces no harmful emissions, making hydrogen fuel cell vehicles a genuinely zero-emission mode of transportation.
So, what are the key advantages that hydrogen-powered vehicles bring to the table? First and foremost, it’s their environmental credentials. By emitting only water vapor as a byproduct, these vehicles contribute to improving air quality and reducing greenhouse gas emissions. This is particularly important in densely populated urban areas where air pollution poses a significant health risk.
Another significant advantage of hydrogen fuel cell vehicles is their extended range. While BEVs have made great strides in range improvement, hydrogen vehicles have the upper hand when it comes to covering long distances. This is because hydrogen can be stored at high energy densities, allowing for more extended ranges without the need for frequent recharging.
Furthermore, refueling a hydrogen vehicle is a breeze, taking just a few minutes, much like refueling a conventional gasoline-powered car. This quick refueling time eliminates the range anxiety that can be a concern for BEV drivers who must plan their journeys around available charging infrastructure.
However, it’s essential to acknowledge the challenges that hydrogen fuel cell technology faces. Chief among them is the need to establish a robust and widespread hydrogen refueling infrastructure. To make hydrogen-powered vehicles a practical choice for consumers, governments and private stakeholders must invest in building a network of hydrogen refueling stations, akin to the extensive network of gasoline and diesel fueling stations we have today.
The cost of hydrogen fuel cell vehicles is another hurdle. Currently, they tend to be more expensive than traditional gasoline-powered cars and even some BEVs. However, with advancements in technology and economies of scale, these costs are expected to decrease over time, making hydrogen vehicles more accessible to a broader range of consumers.
Moreover, the overall efficiency of the hydrogen production, transportation, and conversion process is not as high as that of BEVs, which generate electricity directly from batteries. As the industry continues to refine and optimize these processes, hydrogen fuel cell vehicles should become more energy-efficient.
In conclusion, hydrogen fuel cell technology is emerging as a promising solution in the automotive industry’s quest for sustainable, zero-emission transportation. While it faces challenges in terms of infrastructure, cost, and efficiency, ongoing research, development, and investment efforts hold the potential to overcome these obstacles. As we continue to prioritize sustainability and reduce carbon emissions, hydrogen-powered vehicles are poised to play a pivotal role in shaping the future of clean and eco-friendly transportation.
To delve further into this matter, we encourage you to check out the additional resources provided here: Hydrogen Production and Distribution – Alternative Fuels Data Center
A fuel cell is an electrochemical device that converts the chemical energy of hydrogen and oxygen into electricity, water, and heat. The fundamental principle behind fuel cells is based on the chemical reaction between hydrogen and oxygen to produce electricity without combustion, making it a clean energy source.
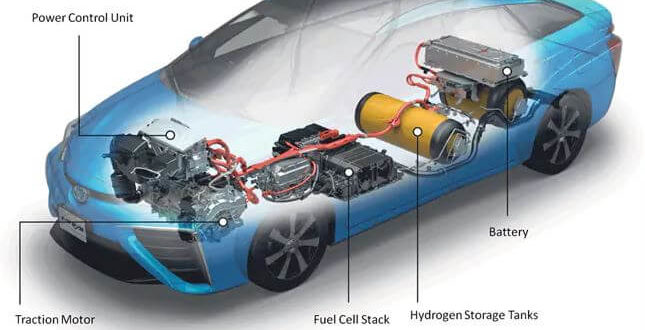
The main components of a hydrogen fuel cell system include:
A fuel cell is an electrochemical device that converts the chemical energy of hydrogen and oxygen into electricity, water, and heat. The fundamental principle behind fuel cells is based on the chemical reaction between hydrogen and oxygen to produce electricity without combustion, making it a clean and sustainable energy source. The main components of a hydrogen fuel cell system include:
Electrolyte: At the heart of every fuel cell is an electrolyte, a material that allows ions (charged particles) to pass through it while blocking the passage of electrons. Different types of fuel cells use various electrolyte materials such as proton-exchange membranes (PEM), solid oxide, and alkaline electrolytes, each with unique advantages and applications.
Anode: On one side of the fuel cell, there is an anode, typically made of a catalyst-coated material. This is where hydrogen molecules (H2) are introduced, and a chemical reaction takes place. At the anode, hydrogen molecules are split into protons (H+) and electrons (e-).
Cathode: On the opposite side of the fuel cell, there is a cathode, also coated with a catalyst. Oxygen molecules (O2) are introduced at the cathode, and they combine with electrons and protons that have traveled through the electrolyte from the anode. This reaction generates water (H2O) as a byproduct.
Electrical Circuit: The electrons produced at the anode cannot pass through the electrolyte, so they travel through an external electrical circuit to reach the cathode. This flow of electrons through the circuit creates an electrical current, which can be harnessed as usable electricity.
Proton Exchange (or Ion Exchange) Membrane: In some types of fuel cells, such as PEM fuel cells, a proton exchange membrane is used as the electrolyte. This membrane selectively allows protons to pass through while blocking electrons, facilitating the separation of the chemical reactions at the anode and cathode.
Bipolar Plates: These plates are located on both sides of the fuel cell stack, and they help distribute gases (hydrogen and oxygen) evenly across the electrodes. Bipolar plates also serve as current collectors, ensuring efficient electron flow within the fuel cell.
Cooling and Thermal Management System: Fuel cells generate heat as a byproduct of the electrochemical reactions. A cooling and thermal management system is essential to regulate the temperature within the fuel cell to maintain optimal performance and prevent overheating.
Gas Supply and Control: To keep the fuel cell operational, a system for supplying and controlling the flow of hydrogen and oxygen to the anode and cathode is required. This includes gas storage tanks, pumps, and controls to maintain the proper gas mixture and pressure.
Balance of Plant Components: Various auxiliary components, such as humidifiers to control moisture levels, filters to remove impurities, and pressure regulators, are part of the balance of plant (BoP) to support the fuel cell system’s overall operation.
Power Electronics and Inverter: Fuel cell-generated electricity is typically direct current (DC). To make it compatible with most electrical devices and the grid, power electronics and an inverter are used to convert DC into alternating current (AC).
Fuel cells have gained attention as a promising clean energy technology due to their high efficiency, low emissions, and versatility. They find applications in various sectors, including transportation (fuel cell vehicles), stationary power generation, backup power systems, and even in space exploration. As research and development continue to advance, fuel cells are likely to play an increasingly important role in the transition to a more sustainable and environmentally friendly energy future.
Additionally, you can find further information on this topic by visiting this page: Use of hydrogen – U.S. Energy Information Administration (EIA)
The hydrogen gas is supplied to the anode (negative electrode), where it is split into protons and electrons.
The hydrogen gas is supplied to the anode (negative electrode), where it is split into protons and electrons, setting in motion a complex yet fascinating series of events that lie at the heart of various scientific and technological applications.
As the hydrogen molecules reach the anode’s surface, they undergo a process known as electrolysis, a fundamental principle in chemistry and electrochemistry. Under the influence of an external electrical potential, the hydrogen gas molecules (H2) are adsorbed onto the anode’s surface, and the process of dissociation begins. This is where the magic happens, as the molecular bonds holding the two hydrogen atoms together are broken.
The first step in this process involves the removal of electrons from the hydrogen molecules. These negatively charged electrons are released and become part of the electrical current flowing through the circuit. This flow of electrons constitutes an electrical current, which can be harnessed for various applications, from powering everyday devices to fueling advanced technological systems.
Simultaneously, the positively charged hydrogen ions, or protons, are released during this dissociation process. These protons migrate through an electrolyte or ion-conductive material toward the cathode (positive electrode) under the influence of the electric field created by the external voltage source. This migration of protons through the electrolyte plays a crucial role in various electrochemical processes, such as in fuel cells and batteries.
The protons’ journey to the cathode is not just a passive drift; it involves a fascinating interplay with other ions and molecules in the electrolyte, leading to chemical reactions that can be harnessed for a wide range of applications. This movement of protons, along with the flow of electrons, forms the basis for various technologies like fuel cells, which efficiently convert the chemical energy of hydrogen into electricity, and water electrolysis, a sustainable method for producing hydrogen gas as a clean energy carrier.
In addition to its scientific significance, the split of hydrogen gas into protons and electrons at the anode has significant implications for clean energy, environmental sustainability, and advancements in energy storage and conversion technologies. It serves as a pivotal step in many electrochemical processes that are driving innovation in fields ranging from renewable energy to transportation, making it a focal point of research and development in the quest for a more sustainable and technologically advanced future.
To delve further into this matter, we encourage you to check out the additional resources provided here: Fuel Cell Basics | Department of Energy

Oxygen or air is supplied to the cathode (positive electrode), where it combines with electrons and protons to form water.
Oxygen or air is supplied to the cathode (positive electrode), where it combines with electrons and protons to form water. This seemingly simple chemical reaction plays a pivotal role in a wide range of essential processes, from powering our electronic devices to enabling life itself.
At its core, this electrochemical reaction is the basis for various applications, one of the most prevalent being in batteries. In rechargeable lithium-ion batteries, for example, oxygen reduction at the cathode is a crucial part of the energy storage process. When the battery discharges, the cathode undergoes a chemical transformation, with oxygen ions reacting with electrons and protons to form water. This conversion of chemical energy into electrical energy is what allows our smartphones, laptops, and electric vehicles to function efficiently.
Furthermore, the principle extends beyond the realm of technology and into the natural world. In the human body, for instance, this reaction takes place in the mitochondria, often referred to as the “powerhouses” of our cells. Here, oxygen combines with electrons and protons during cellular respiration, producing water as a byproduct while generating the energy needed for various biological processes. This essential metabolic activity ensures our cells receive the energy required to sustain life.
Beyond batteries and biology, the oxygen reduction reaction holds immense promise in sustainable energy technologies. Hydrogen fuel cells, for instance, rely on this reaction to produce electricity with water as the only byproduct, offering a clean and efficient alternative to fossil fuels. This technology has the potential to revolutionize transportation and energy production, reducing greenhouse gas emissions and mitigating climate change.
In summary, the seemingly straightforward process of supplying oxygen or air to the cathode, where it combines with electrons and protons to form water, has profound implications across various domains. From powering our devices to sustaining life and driving advancements in clean energy, this electrochemical reaction serves as a cornerstone for innovation and progress in the modern world.
Additionally, you can find further information on this topic by visiting this page: Fuel Cells: A Better Energy Source for Earth and Space – NASA

An electrolyte membrane separates the anode and cathode and allows the movement of protons while preventing the mixing of hydrogen and oxygen gases.
An electrolyte membrane, often at the heart of fuel cells and various electrochemical devices, serves as an indispensable barrier within the electrochemical cell. This critical component is not just a passive divider; it plays a dynamic role in ensuring the efficient and safe operation of these systems.
First and foremost, the electrolyte membrane serves as a selective conductor, enabling the controlled movement of protons (H+ ions) while obstructing the uncontrolled mixing of hydrogen (H2) and oxygen (O2) gases. This selective permeability is what differentiates the electrolyte membrane from a simple physical barrier. Protons, the key players in the electrochemical reactions within the cell, traverse the membrane, facilitated by its unique properties, to participate in the reactions that generate electrical energy.
Moreover, the electrolyte membrane is a multifaceted guardian of the cell’s integrity. It not only separates the anode and cathode but also prevents the disastrous blending of hydrogen and oxygen gases. This is particularly crucial, as the combination of these two gases in uncontrolled conditions can lead to explosive consequences. By effectively segregating the reactants, the electrolyte membrane acts as a safety mechanism, ensuring that only controlled, electrochemical reactions take place within the cell.
In addition to its role in safety and ion transport, the electrolyte membrane can impact the overall performance and efficiency of the electrochemical device. Its design and material composition can influence factors such as conductivity, durability, and resistance to contaminants. Innovations in electrolyte membrane technology are continuously pursued to enhance the performance and longevity of devices, making them more sustainable and cost-effective.
Furthermore, the choice of electrolyte membrane can vary depending on the specific application. Proton Exchange Membrane (PEM) fuel cells employ a different type of electrolyte membrane compared to Solid Oxide Fuel Cells (SOFCs) or other electrochemical systems. Each type of membrane has its advantages and limitations, making the selection of the appropriate membrane a critical aspect of designing electrochemical devices.
In summary, the electrolyte membrane in electrochemical cells is not merely a physical divider but a dynamic and versatile component. It selectively conducts protons, safeguards against potentially hazardous gas mixing, influences device performance, and necessitates careful consideration during the design and operation of electrochemical systems. Its crucial role underscores the significance of ongoing research and innovation in the field of membrane materials and technologies, paving the way for more efficient, sustainable, and safer electrochemical devices in various applications.
Explore this link for a more extensive examination of the topic: Fuel Cell Electric Vehicles – Alternative Fuels Data Center

Platinum or other noble metals are typically used as catalysts to facilitate the reactions at the anode and cathode.
Platinum and other noble metals have long been the stalwarts of catalysis, playing a pivotal role in various chemical processes, particularly those involving electrochemical reactions at the anode and cathode. However, as we delve deeper into the world of materials science and sustainable technologies, it becomes evident that the exclusivity of noble metals might not be the sustainable or economically viable path forward.
While platinum has exhibited unparalleled catalytic properties, its scarcity and high cost have raised concerns about the feasibility of scaling up technologies that heavily rely on it. Fortunately, researchers are exploring alternative catalyst materials that can not only mimic the performance of noble metals but also address these issues.
One promising avenue is the development of non-noble metal catalysts, often derived from earth-abundant elements like iron, nickel, or cobalt. These materials have shown remarkable potential in accelerating electrochemical reactions, offering a more sustainable and cost-effective alternative to platinum. For instance, iron-based catalysts have gained traction in the oxygen reduction reaction, a crucial process in fuel cells and metal-air batteries.
Moreover, advancements in nanotechnology and nanomaterials have revolutionized the field of catalysis. Nanostructured catalysts, made from a variety of materials including non-noble metals, have demonstrated exceptional activity and selectivity due to their high surface area and unique electronic properties. These innovations are paving the way for more efficient and eco-friendly electrochemical systems.
Furthermore, the integration of advanced computational methods and artificial intelligence is accelerating catalyst discovery. Researchers can now screen vast databases of materials to identify potential candidates for catalytic applications, reducing the time and resources required for experimentation.
In addition to these material-centric approaches, the design of novel electrode architectures and engineering of electrolytes are also playing crucial roles in enhancing the performance of electrochemical systems. These innovations complement the development of alternative catalysts, collectively contributing to more sustainable and efficient energy conversion and storage technologies.
In conclusion, while platinum and noble metals have long been catalysts of choice for electrochemical reactions, the future holds promise for a more diversified and sustainable landscape. The quest for alternative materials, coupled with advancements in nanotechnology, computational modeling, and system engineering, is reshaping the world of catalysis, opening up new possibilities for cleaner energy production and storage. As we continue to explore these frontiers, we may find ourselves relying less on the exclusivity of noble metals and more on innovative solutions that benefit both our planet and our wallets.
For additional details, consider exploring the related content available here Platinum-free catalysts could make cheaper hydrogen fuel cells …

Hydrogen fuel cell vehicles produce no tailpipe emissions. The only byproduct of the chemical reaction is water vapor, making them a truly clean and environmentally friendly transportation option.
Hydrogen fuel cell vehicles represent a significant leap forward in sustainable transportation technology, offering numerous advantages beyond their zero tailpipe emissions. By harnessing the power of hydrogen gas and oxygen in a chemical reaction to generate electricity to power the vehicle’s electric motor, these vehicles are poised to revolutionize the way we think about clean mobility.
Energy Efficiency: Hydrogen fuel cell vehicles are highly efficient in converting chemical energy into electricity and, ultimately, into motion. This efficiency is achieved by bypassing the intermediate step of converting hydrogen into electricity via combustion, as is the case with internal combustion engines. As a result, hydrogen fuel cell vehicles have the potential to outperform many traditional gasoline or diesel-powered vehicles in terms of energy efficiency.
Reduced Dependence on Fossil Fuels: One of the most pressing global challenges is reducing our dependence on fossil fuels to combat climate change. Hydrogen fuel cell vehicles can play a pivotal role in this transition. Hydrogen can be produced from a variety of sources, including renewable resources like wind, solar, and biomass, or even from excess electricity during off-peak hours. This flexibility in hydrogen production methods allows for a cleaner and more sustainable energy ecosystem.
Fast Refueling and Long Range: Hydrogen fuel cell vehicles offer the convenience of fast refueling times, similar to traditional gasoline vehicles. This stands in contrast to battery electric vehicles (BEVs), which typically have longer charging times. Additionally, hydrogen fuel cell vehicles often have longer ranges compared to BEVs, making them suitable for long-distance travel without the range anxiety commonly associated with electric cars.
Reduced Noise Pollution: Hydrogen fuel cell vehicles are quieter than their internal combustion engine counterparts. The absence of noisy engine components and the smooth operation of electric motors contribute to a quieter, more pleasant urban environment. This reduction in noise pollution can have positive effects on public health and quality of life in urban areas.
Potential for Energy Storage and Grid Stabilization: Beyond transportation, hydrogen has the potential to serve as an energy storage medium. Excess electricity from renewable sources can be used to produce hydrogen, which can then be stored and used to generate electricity when demand is high or renewable sources are unavailable. This could help stabilize the electrical grid and facilitate the integration of more renewable energy into the power supply.
Innovations in Green Hydrogen Production: As technology continues to advance, there is growing potential for the widespread production of “green” hydrogen, which is produced using renewable energy sources and emits no greenhouse gases during production. The development of efficient and cost-effective green hydrogen production methods will further enhance the environmental credentials of hydrogen fuel cell vehicles.
In summary, hydrogen fuel cell vehicles are not only a solution for zero tailpipe emissions but also represent a multifaceted approach to creating a more sustainable and environmentally friendly transportation system. Their energy efficiency, ability to reduce fossil fuel dependence, convenience, and potential for broader energy applications make them a promising contender in the quest for a greener future.
Looking for more insights? You’ll find them right here in our extended coverage: Hydrogen Fuel Cell Electric Cars | DriveClean

Hydrogen vehicles typically have a longer driving range compared to BEVs. This is because hydrogen can be stored at higher energy densities than batteries, allowing for greater travel distances between refueling.
Hydrogen vehicles, with their longer driving range compared to Battery Electric Vehicles (BEVs), showcase a promising future for sustainable transportation. This extended range is primarily attributed to the fundamental differences in energy storage between hydrogen and batteries. Here, we’ll delve deeper into the factors that contribute to this advantage and explore the broader implications for the future of transportation:
Energy Density: Hydrogen boasts an impressive energy density, far surpassing the capabilities of even the most advanced batteries available today. This means that a relatively small volume of hydrogen can store a significant amount of energy, which translates to more miles on the road before refueling becomes necessary. This characteristic makes hydrogen vehicles particularly appealing for long-distance travel, where convenience and range anxiety are critical concerns for consumers.
Quick Refueling: Unlike the lengthy charging times associated with BEVs, hydrogen vehicles offer a refueling experience similar to traditional gasoline or diesel vehicles. Filling up a hydrogen tank takes just a few minutes, providing a level of convenience and familiarity that can be a game-changer for consumers. This quick refueling option encourages the adoption of hydrogen vehicles, especially for those who cannot afford to wait for extended charging periods during their busy daily routines.
Weight and Efficiency: Hydrogen fuel cells tend to be more energy-efficient than traditional internal combustion engines. They convert a higher percentage of the energy contained in the fuel into usable power for the vehicle, resulting in reduced energy waste. Additionally, hydrogen tanks are typically lighter than large battery packs, contributing to overall vehicle weight reduction. This weight advantage further enhances hydrogen vehicles’ driving range, as lighter vehicles require less energy to operate.
Versatile Applications: Hydrogen’s unique characteristics extend beyond just passenger vehicles. It can also be used in various transportation modes, including buses, trucks, and even trains. These applications can benefit significantly from the extended range offered by hydrogen, making it a viable option for freight transport and mass transit systems. Consequently, hydrogen has the potential to revolutionize not only personal mobility but also the broader transport industry.
Infrastructure Challenges: While the advantages of hydrogen are clear, it’s important to acknowledge the infrastructure challenges that still need to be addressed. Building a network of hydrogen refueling stations is a complex and costly endeavor, especially when compared to the relatively simpler task of expanding the electric charging infrastructure. Overcoming these challenges will be crucial to realizing the full potential of hydrogen vehicles.
In conclusion, the longer driving range of hydrogen vehicles compared to BEVs is a compelling argument in favor of their continued development and adoption. The energy density of hydrogen, coupled with quick refueling, efficiency, and versatility, positions hydrogen as a key player in the future of sustainable transportation. However, addressing infrastructure limitations is essential to fully harness the benefits of hydrogen-powered vehicles and pave the way for a cleaner, more efficient, and convenient transportation future.
Additionally, you can find further information on this topic by visiting this page: Hydrogen Fuel Cell Electric Cars | DriveClean

Refueling a hydrogen-powered vehicle takes only a few minutes, similar to filling up a conventional gasoline car. This contrasts with the longer charging times required for BEVs.
Refueling a hydrogen-powered vehicle takes only a few minutes, similar to filling up a conventional gasoline car. This speedy refueling process stands as one of the most compelling advantages of hydrogen vehicles and addresses a critical concern that many potential electric vehicle (EV) owners have: charging time.
When it comes to battery electric vehicles (BEVs), one of the primary concerns has been the time it takes to recharge their batteries. Even with the rapid advancements in EV charging infrastructure and technologies, charging a BEV can still take significantly longer than refueling a hydrogen vehicle or a traditional gasoline-powered car.
For instance, even with high-power Level 3 DC fast chargers, BEVs often require around 30 minutes to charge up to 80% of their battery capacity. While this is acceptable for many daily commuting needs and short trips, it can become less convenient for longer journeys. It’s a stark contrast to the quick refueling experience that hydrogen vehicles offer, where you can replenish the hydrogen tank in a matter of minutes, much like the familiar process of filling up with gasoline.
This difference in refueling times can be particularly advantageous for drivers who cover long distances regularly, such as commercial truckers, who can save valuable time on the road by opting for hydrogen-powered vehicles. Additionally, quick refueling can make hydrogen vehicles more appealing to consumers accustomed to the convenience of traditional gasoline vehicles, as it eliminates the need for planning extended stops to charge.
However, it’s essential to acknowledge that the charging infrastructure for BEVs is continually improving. The widespread deployment of fast-charging stations, along with advancements in battery technology, is gradually reducing the time it takes to charge electric vehicle batteries. In the near future, BEV charging times may become even more competitive with hydrogen refueling.
Another notable aspect of hydrogen refueling is its similarity to the existing gasoline refueling infrastructure. Hydrogen stations are designed to be easily accessible and familiar to consumers, making the transition from gasoline vehicles to hydrogen vehicles more seamless. This infrastructure advantage can contribute to the broader adoption of hydrogen-powered vehicles, especially as hydrogen refueling networks continue to expand.
In conclusion, the rapid refueling experience of hydrogen-powered vehicles compared to the longer charging times of BEVs is a significant selling point for hydrogen technology. It addresses a key concern for consumers and commercial drivers who value the convenience of quick refueling. While BEVs are making progress in reducing charging times, the efficient and familiar hydrogen refueling process is a compelling factor that adds to the overall appeal of hydrogen-powered vehicles in the evolving landscape of sustainable transportation.
Explore this link for a more extensive examination of the topic: Hydrogen Fuel Cell Electric Cars | DriveClean

Hydrogen can be produced from various renewable sources, such as wind, solar, or biomass, providing flexibility in its sourcing and reducing dependency on fossil fuels.
Hydrogen can be produced from various renewable sources, such as wind, solar, or biomass, providing flexibility in its sourcing and reducing dependency on fossil fuels. This dynamic aspect of hydrogen production holds the key to a more sustainable and environmentally friendly energy landscape. Here’s an extended exploration of this idea:
Wind-Powered Hydrogen Production: Wind turbines can be used to generate electricity, which can then be used in the electrolysis process to split water (H2O) into hydrogen (H2) and oxygen (O2). This approach, often referred to as “wind-to-hydrogen,” offers a means of storing excess energy generated during periods of high wind availability. This stored hydrogen can later be used for electricity generation or as a clean fuel for various applications, such as fuel cell vehicles.
Solar-Powered Hydrogen Production: Solar panels can harness sunlight to produce electricity, which can be used for electrolysis or other hydrogen production methods. Solar-to-hydrogen systems are particularly appealing in regions with abundant sunshine. Furthermore, advancements in photovoltaic technology, such as perovskite solar cells, hold promise for more efficient and cost-effective hydrogen production.
Biomass Gasification: Biomass, including organic waste, agricultural residues, and dedicated energy crops, can be converted into hydrogen-rich gas through a process called gasification. This gas can then be purified to extract hydrogen. Biomass-based hydrogen production not only offers a renewable and carbon-neutral hydrogen source but also helps in waste management and reduces greenhouse gas emissions.
Electrolysis with Renewable Electricity: Regardless of the energy source, the key to sustainable hydrogen production lies in using renewable electricity for the electrolysis process. As the share of renewables in the energy mix grows, the carbon footprint of hydrogen production decreases. Moreover, excess renewable energy can be stored as hydrogen and released when needed, providing grid stability.
Hydrogen as an Energy Carrier: Hydrogen’s versatility makes it an ideal energy carrier. It can be transported and stored relatively easily, addressing the intermittent nature of some renewable sources. When renewables produce excess energy, such as during sunny or windy days, this surplus can be used to produce hydrogen. Later, when renewables are less available, hydrogen can be used for electricity generation or as a clean fuel for industrial processes and transportation.
Reducing Fossil Fuel Dependency: By expanding the use of renewable hydrogen, we reduce our dependence on fossil fuels for energy production and transportation. This has significant environmental and energy security benefits, as it lowers carbon emissions and mitigates the geopolitical challenges associated with fossil fuel imports.
Green Hydrogen: Hydrogen produced using renewable sources is often referred to as “green hydrogen.” It has the potential to play a crucial role in achieving carbon neutrality and decarbonizing sectors that are difficult to electrify fully, such as heavy industry and long-haul transportation. Governments and industries around the world are investing in green hydrogen initiatives to accelerate its adoption.
Technological Advancements: Ongoing research and development are focused on improving the efficiency and cost-effectiveness of hydrogen production from renewable sources. Innovations in electrolysis technologies, catalysts, and storage solutions are driving the growth of the renewable hydrogen sector.
In conclusion, the ability to produce hydrogen from renewable sources offers a promising pathway to a more sustainable and decarbonized energy future. This approach not only helps reduce greenhouse gas emissions but also enhances energy security and resilience by diversifying our energy sources. As renewable energy capacity continues to expand, so does the potential for green hydrogen as a key component of a clean and resilient energy system.
For a comprehensive look at this subject, we invite you to read more on this dedicated page: The Potential of Hydrogen Fuel Cells in Energy Storage for EV …
While hydrogen fuel cell technology offers many advantages, it also faces several challenges:
While hydrogen fuel cell technology offers many advantages, it also faces several challenges that must be addressed to realize its full potential as a clean and efficient energy solution for various applications.
Hydrogen Production and Distribution: One of the primary challenges is the production and distribution of hydrogen. Currently, the majority of hydrogen is produced from fossil fuels, primarily natural gas, through a process called steam methane reforming. Transitioning to cleaner methods of hydrogen production, such as electrolysis using renewable energy sources, is crucial to reduce the carbon footprint of hydrogen production.
Energy Efficiency: Hydrogen fuel cells are relatively efficient at converting hydrogen into electricity, but there are energy losses at various stages of the process, including hydrogen production, compression, and distribution. Improving the overall energy efficiency of the entire hydrogen supply chain is a significant challenge.
Storage: Hydrogen has a low volumetric energy density, which makes it challenging to store and transport efficiently. Finding safe, cost-effective, and compact methods for hydrogen storage is a key challenge for the widespread adoption of hydrogen as an energy carrier.
Cost: Hydrogen fuel cells are currently more expensive than some other energy storage and conversion technologies, such as batteries. Reducing the cost of manufacturing fuel cells and associated components is essential to make hydrogen technology competitive in various markets.
Infrastructure Development: Establishing a comprehensive hydrogen infrastructure, including refueling stations and pipelines, is a significant challenge. This infrastructure must be scalable and adaptable to accommodate the growing demand for hydrogen in various sectors, from transportation to industry.
Safety Concerns: Hydrogen is highly flammable and can be challenging to handle safely, particularly in densely populated areas or confined spaces. Developing and implementing rigorous safety standards and practices is essential to ensure the safe use of hydrogen fuel cells.
Material Durability: The materials used in fuel cells, such as catalysts and membranes, can degrade over time, reducing the lifespan and performance of the cells. Research into more durable and cost-effective materials is ongoing.
Scale-Up and Integration: Scaling up hydrogen fuel cell technology to meet the demands of large-scale applications, such as grid energy storage or long-haul transportation, is a significant challenge. Integration with existing energy systems and infrastructure also requires careful planning and investment.
Public Perception and Education: Building public awareness and acceptance of hydrogen as a clean energy solution is crucial. Addressing misconceptions and educating the public about the benefits and safety of hydrogen technology is an ongoing challenge.
Despite these challenges, ongoing research, development, and innovation in the field of hydrogen fuel cell technology are steadily overcoming these obstacles. As governments, industries, and researchers work together, hydrogen has the potential to play a vital role in a sustainable and low-carbon energy future, offering clean, reliable, and versatile energy solutions.
Don’t stop here; you can continue your exploration by following this link for more details: Hydrogen Benefits and Considerations – Alternative Fuels Data Center

One of the most significant hurdles is the limited infrastructure for hydrogen refueling stations. Building a comprehensive network of refueling stations is essential for the widespread adoption of hydrogen-powered vehicles.
One of the most significant hurdles is the limited infrastructure for hydrogen refueling stations. Building a comprehensive network of refueling stations is essential for the widespread adoption of hydrogen-powered vehicles. This challenge not only presents obstacles but also offers opportunities for innovation, collaboration, and the transformation of our transportation systems and energy landscape.
Innovation in Refueling Technology: To overcome the infrastructure gap, researchers and engineers are actively working on developing advanced refueling technologies. This includes fast-fill stations, where vehicles can refuel as quickly as traditional gasoline vehicles, and even home-based refueling solutions, allowing consumers to produce hydrogen on-site through electrolysis. These innovations can expedite the growth of the hydrogen refueling infrastructure.
Public-Private Partnerships: Governments, industries, and environmental organizations are increasingly recognizing the potential of hydrogen as a clean energy carrier. This realization has prompted the formation of public-private partnerships aimed at investing in and expanding hydrogen refueling networks. These collaborations can accelerate the development of critical infrastructure.
Multi-Purpose Stations: Another approach to expanding the hydrogen infrastructure involves multi-purpose stations. These facilities could offer not only hydrogen refueling but also other energy-related services such as battery charging, natural gas dispensing, or renewable energy generation. By diversifying their offerings, these stations can become financially sustainable and more appealing to investors.
Strategic Location Planning: Identifying strategic locations for refueling stations is vital. Initially targeting urban areas, major highways, and transportation hubs can help create the foundation of a hydrogen refueling network. Over time, expansion into rural and remote areas can ensure that hydrogen-powered vehicles are viable options for people regardless of their location.
Education and Awareness: Public awareness and understanding of hydrogen as a fuel source are crucial. Governments and organizations can undertake educational campaigns to inform consumers about the benefits of hydrogen-powered vehicles and the availability of refueling infrastructure. This can create demand, which in turn can encourage private investment in infrastructure development.
Hydrogen Production and Supply Chain: A robust hydrogen supply chain is essential for the success of refueling stations. Innovations in sustainable hydrogen production methods, such as electrolysis powered by renewable energy sources, can help ensure a consistent and environmentally friendly supply of hydrogen to these stations.
Regulatory Support: Governments can play a pivotal role in fostering the growth of hydrogen infrastructure by providing incentives, subsidies, and regulatory frameworks that support the development and operation of refueling stations. These measures can attract private investment and accelerate expansion.
In conclusion, while the limited infrastructure for hydrogen refueling stations poses a significant challenge, it also serves as a catalyst for technological innovation and collaboration. Overcoming this hurdle is not only essential for the widespread adoption of hydrogen-powered vehicles but also holds the potential to revolutionize our transportation systems and reduce greenhouse gas emissions on a global scale. By addressing these challenges proactively and embracing new opportunities, we can pave the way for a sustainable and hydrogen-powered future.
Don’t stop here; you can continue your exploration by following this link for more details: Fuel Cell Electric Vehicles – Alternative Fuels Data Center

Producing and transporting hydrogen can be energy-intensive and may involve emissions if not sourced from renewable energy. Finding sustainable and cost-effective hydrogen production methods is critical.
Producing and transporting hydrogen, often referred to as the “energy carrier of the future,” indeed holds great promise in the transition towards a more sustainable energy landscape. However, it’s important to recognize that the process is not without its challenges and environmental considerations. To ensure the widespread adoption of hydrogen as a clean energy source, finding sustainable and cost-effective hydrogen production methods is not just desirable but imperative.
Green Hydrogen Production: Green hydrogen production, generated through the process of electrolysis using renewable energy sources like wind or solar power, is a beacon of hope for sustainability. This method involves splitting water molecules into hydrogen and oxygen, emitting zero greenhouse gases when renewable energy is used. As the renewable energy sector continues to grow, so does the potential for clean, sustainable hydrogen production.
Efficiency Improvement: Advancements in electrolysis technology are crucial to enhance the energy efficiency of hydrogen production. Electrolyzers with higher conversion efficiencies reduce the energy input required, making hydrogen production more sustainable. Additionally, research into new catalysts and materials can further optimize the electrolysis process, lowering its environmental impact.
Carbon Capture and Utilization: Hydrogen production from fossil fuels can be made more sustainable by implementing carbon capture and utilization (CCU) technologies. These systems capture carbon emissions produced during hydrogen production and repurpose them for other industrial applications, mitigating their impact on the environment.
Nuclear and Advanced Thermal Processes: Nuclear energy and advanced thermal processes like high-temperature steam electrolysis can also provide a sustainable pathway for hydrogen production. These methods can yield high-purity hydrogen with minimal greenhouse gas emissions, provided safety and waste disposal challenges are effectively addressed.
Hydrogen Transportation: Transporting hydrogen efficiently is another key aspect. Innovative storage and transportation solutions, such as advanced composite materials for high-pressure tanks or cryogenic storage methods, can reduce energy losses during transportation and ensure that hydrogen arrives at its destination with minimal energy wastage.
Hydrogen Pipelines: The development of dedicated hydrogen pipelines can significantly reduce transportation energy costs. Establishing a network of pipelines can facilitate the movement of hydrogen from production centers to end-users, similar to existing natural gas infrastructure.
Hydrogen Production from Waste: Utilizing waste streams, such as organic matter or industrial byproducts, for hydrogen production presents an opportunity to reduce both waste disposal and hydrogen production costs. Technologies like gasification and biogas reforming can extract hydrogen from waste materials.
International Collaboration: Given the global nature of hydrogen markets, international collaboration is essential. Sharing best practices, research findings, and standards can accelerate the development of sustainable and cost-effective hydrogen production methods on a global scale.
In conclusion, the journey towards sustainable and cost-effective hydrogen production is an ongoing and multifaceted effort. It involves innovation across various technologies, energy sources, and infrastructure development. By addressing these challenges and leveraging emerging technologies, we can unlock the full potential of hydrogen as a clean, versatile, and environmentally friendly energy carrier, contributing to a more sustainable future.
If you’d like to dive deeper into this subject, there’s more to discover on this page: Hydrogen in Transportation | US EPA

Hydrogen fuel cell vehicles are currently more expensive than their gasoline and battery electric counterparts. Economies of scale and advancements in technology may help reduce costs over time.
Hydrogen fuel cell vehicles, despite their promising potential as a clean and efficient transportation solution, currently face cost challenges that make them less competitive when compared to traditional gasoline and battery electric vehicles. However, there is optimism that economies of scale and ongoing technological advancements will gradually narrow this cost gap, making hydrogen fuel cell vehicles a more attractive and accessible option in the future.
Economies of Scale: One of the primary factors driving up the cost of hydrogen fuel cell vehicles today is the limited scale of production. As the demand for these vehicles increases, manufacturers can benefit from economies of scale. Large-scale production enables more efficient manufacturing processes, bulk purchasing of materials, and streamlined supply chains, all of which can significantly reduce production costs. This has already started to happen in regions where hydrogen infrastructure and demand are growing, such as Japan and parts of Europe.
Advancements in Technology: Ongoing research and development efforts are continuously improving the efficiency and durability of fuel cell technology. Innovations in catalyst materials, membrane technology, and system design are making fuel cells more energy-efficient and longer-lasting. As these technologies mature and become more cost-effective, they will contribute to lowering the overall cost of hydrogen fuel cell systems.
Infrastructure Development: The availability and accessibility of hydrogen refueling stations are critical to the adoption of fuel cell vehicles. Increased investments in hydrogen infrastructure will not only make it more convenient for consumers to use these vehicles but also stimulate greater demand, further driving economies of scale. Governments and private industry are recognizing the importance of this infrastructure and are working to expand it.
Complementary Applications: Hydrogen fuel cells have applications beyond just transportation, including power generation and industrial processes. As the hydrogen economy grows, the production of hydrogen for various uses can become more efficient and cost-effective. This can, in turn, lead to cost reductions in the automotive sector as the cost of hydrogen production decreases.
Market Competition: As more automakers invest in hydrogen fuel cell technology and enter the market, competition will intensify. This competition can lead to innovations and cost reductions as companies strive to gain a competitive edge by offering more affordable and advanced fuel cell vehicles.
Government Support: Many governments worldwide are providing incentives and subsidies to encourage the development and adoption of hydrogen fuel cell vehicles. These policies can help offset the initial cost premium and stimulate demand, which, in turn, can lead to cost reductions through increased production.
In summary, while hydrogen fuel cell vehicles are currently more expensive than their gasoline and battery electric counterparts, the future holds promise. Economies of scale, ongoing technological advancements, infrastructure development, market competition, and government support are all factors working together to make hydrogen fuel cell vehicles more affordable and accessible. As these factors align, we can expect to see a gradual reduction in the cost disparity, making hydrogen-powered transportation a more competitive and environmentally friendly choice in the automotive market.
Explore this link for a more extensive examination of the topic: Hydrogen’s Role in Transportation | Department of Energy

Hydrogen production, transportation, and conversion in fuel cells involve energy losses at each step, making the overall process less efficient compared to direct electricity generation in BEVs.
While it’s true that the hydrogen production, transportation, and conversion process in fuel cell vehicles does involve energy losses at each stage, it’s important to acknowledge the complexities and evolving dynamics of this technology. Here are some considerations that provide a more nuanced perspective on the efficiency of hydrogen fuel cell vehicles compared to battery electric vehicles (BEVs):
Advancements in Electrolysis: The process of producing hydrogen, often through electrolysis of water, has seen significant advancements. New technologies and materials are being developed to make electrolysis more energy-efficient, and some methods even use surplus renewable energy during periods of low demand to produce hydrogen. As these technologies mature, the energy losses in hydrogen production are likely to decrease, improving the overall efficiency of the hydrogen fuel chain.
Transportation Efficiency: While hydrogen transport can involve energy losses, advancements in high-pressure storage and transportation methods have helped reduce these losses. Additionally, the development of localized hydrogen production facilities can minimize the need for long-distance transportation, further improving the energy efficiency of the hydrogen supply chain.
Diverse Applications: Hydrogen’s versatility extends beyond transportation. It can be used in various sectors, including industry and heating, where direct electrification is challenging. This diversity in applications can justify some of the energy losses in the hydrogen supply chain, as it enables the utilization of renewable hydrogen in multiple sectors, contributing to overall energy system decarbonization.
Energy Storage and Grid Integration: Hydrogen can serve as a form of energy storage, allowing excess renewable energy to be stored and used when needed. This is particularly valuable in regions with variable renewable energy sources. While there are losses associated with converting electricity to hydrogen and back to electricity, this capability contributes to grid stability and renewable energy integration.
Long-Range and Heavy-Duty Applications: Hydrogen fuel cell vehicles excel in long-range and heavy-duty applications where the weight and size of large batteries would be impractical. For instance, hydrogen-powered trucks, buses, and trains can offer substantial environmental benefits while meeting the demands of their respective industries.
Hydrogen Blending: Hydrogen can be blended with natural gas in pipelines, reducing the carbon footprint of natural gas use. This approach, known as hydrogen blending, can be a transitional step towards a more sustainable energy mix.
In summary, while it’s true that the hydrogen production and fuel cell conversion process involves energy losses, the evolving landscape of hydrogen technology is continuously improving efficiency. Additionally, the unique capabilities of hydrogen, such as its suitability for long-range and heavy-duty applications and its role in energy storage and grid integration, make it a valuable component of a sustainable energy ecosystem alongside battery electric vehicles. Ultimately, the choice between hydrogen and BEVs will depend on specific use cases and regional factors, with both technologies playing roles in the transition to a cleaner energy future.
You can also read more about this here: What are the Pros and Cons of Hydrogen Fuel Cells? – TWI

As of my last knowledge update in September 2021, hydrogen-powered vehicles were still in the early stages of commercialization, with a few automakers, such as Toyota, Hyundai, and Honda, offering fuel cell vehicles in select markets. However, adoption remained limited due to the challenges mentioned earlier.
It’s worth noting that developments in the field of hydrogen fuel cell technology and infrastructure may have occurred since then. Governments and industries around the world have been investing in research and development to overcome the obstacles associated with hydrogen-powered transportation.
Since my last knowledge update in September 2021, significant advancements and shifts have likely occurred in the landscape of hydrogen-powered vehicles. These developments underscore the ongoing commitment of governments and industries to explore hydrogen as a viable and sustainable energy source for transportation. Here’s an extended overview of the progress made in the realm of hydrogen-powered vehicles:
Wider Adoption and Market Expansion: The commercialization of hydrogen-powered vehicles has likely gained momentum. More automakers may have joined the ranks of Toyota, Hyundai, and Honda in offering fuel cell vehicles, expanding their availability to a broader range of markets. This increased competition could have driven innovation and price reductions, making hydrogen vehicles more accessible to consumers.
Infrastructure Growth: One of the primary challenges facing hydrogen adoption has been the limited refueling infrastructure. Over the years, governments and private entities have likely invested heavily in building a more extensive network of hydrogen refueling stations. This expansion is crucial for encouraging consumers to consider hydrogen vehicles as a practical alternative to traditional gasoline and electric cars.
Technological Improvements: Research and development efforts in hydrogen fuel cell technology have continued unabated. These efforts have likely led to advancements in fuel cell efficiency, durability, and cost-effectiveness. As a result, fuel cells may have become more reliable and affordable, further enhancing the appeal of hydrogen vehicles.
Fleet Applications: Hydrogen’s extended range and quick refueling make it an attractive option for commercial and industrial applications. Delivery trucks, buses, and even trains may have increasingly adopted hydrogen fuel cell technology, contributing to reduced emissions and improved sustainability in the transportation sector.
Global Initiatives: Countries worldwide have continued to prioritize hydrogen as a clean energy solution. Governments have introduced incentives, subsidies, and regulations to promote the adoption of hydrogen-powered vehicles and stimulate investment in related industries. These initiatives have likely accelerated the growth of the hydrogen economy.
Green Hydrogen: The production of “green” hydrogen, which is generated using renewable energy sources like wind and solar power, has gained traction. Green hydrogen is considered more environmentally friendly than hydrogen produced from fossil fuels. The shift toward green hydrogen production could further enhance the environmental benefits of hydrogen-powered transportation.
Collaborative Efforts: International collaborations and partnerships between automakers, energy companies, and research institutions have likely strengthened. These collaborations aim to address shared challenges, share knowledge, and accelerate the development of hydrogen technologies.
In conclusion, the landscape of hydrogen-powered vehicles has likely evolved significantly since my last knowledge update in September 2021. Increased adoption, infrastructure expansion, technological advancements, and growing global support indicate a promising future for hydrogen as a clean and sustainable energy source in the transportation sector. Continued investments and collaborative efforts will be pivotal in realizing the full potential of hydrogen-powered vehicles and reducing greenhouse gas emissions from the transportation industry.
For additional details, consider exploring the related content available here The Future of Hydrogen – Analysis – IEA

Conclusion
Hydrogen fuel cell technology holds great promise for the future of sustainable transportation, offering zero-emission vehicles with longer ranges and quick refueling times. However, significant challenges related to infrastructure, cost, and efficiency need to be addressed for wider adoption. The progress in hydrogen-powered vehicles will depend on continued research, investment, and collaboration between governments, industries, and researchers. As we move towards a more sustainable future, hydrogen-powered vehicles are likely to play a significant role in reducing greenhouse gas emissions in the automotive sector.
Hydrogen fuel cell technology is not just a promising option; it is a crucial piece of the puzzle in the quest for sustainable transportation in the years to come. This revolutionary technology represents a potential game-changer, offering a trifecta of advantages that align with our aspirations for a cleaner, more efficient, and environmentally friendly transportation system.
First and foremost, the zero-emission nature of hydrogen fuel cell vehicles contributes significantly to our efforts to combat climate change and reduce air pollution. With the increasing urgency of global climate goals, transitioning to vehicles that produce only water vapor as their tailpipe emissions can play a pivotal role in achieving a more sustainable future.
One of the most noteworthy features of hydrogen vehicles is their extended range. This attribute is especially pertinent as we envision a future where long-distance travel remains essential. With the ability to cover longer distances between refueling compared to battery electric vehicles (BEVs), hydrogen vehicles become a practical choice for various applications, from cross-country road trips to commercial transportation.
Quick refueling is another significant advantage, and this convenience can make hydrogen vehicles more accessible and appealing to a broader spectrum of consumers. The familiar and efficient refueling experience, akin to that of conventional gasoline cars, can ease the transition to hydrogen-powered transportation.
Nonetheless, it’s important to address the substantial challenges that currently stand in the way of widespread adoption. The first hurdle is the need for a comprehensive and accessible hydrogen refueling infrastructure. Governments, industries, and stakeholders must collaborate to accelerate the deployment of hydrogen refueling stations to ensure that consumers can confidently adopt this technology, knowing that refueling will be convenient and readily available.
Cost is another area that demands attention. Hydrogen fuel cell vehicles are still relatively expensive compared to conventional gasoline vehicles, primarily due to the cost of the fuel cell stack and the limited production scale. However, with sustained research, development, and increased production volumes, we can expect the cost to decrease over time, making these vehicles more affordable for a broader range of consumers.
Efficiency is a concern as well, particularly in the production and transportation of hydrogen. To make hydrogen fuel cell technology more sustainable, it’s essential to optimize these processes and reduce energy losses at each stage. Additionally, exploring green hydrogen production methods, such as electrolysis powered by renewable energy sources, can enhance the overall environmental benefits of hydrogen vehicles.
In conclusion, the realization of hydrogen-powered vehicles’ full potential hinges on continued research, investment, and collaboration across sectors. Governments, industries, and researchers must work hand in hand to overcome the challenges related to infrastructure, cost, and efficiency. As we navigate towards a more sustainable future, hydrogen-powered vehicles are poised to play a pivotal role in curbing greenhouse gas emissions and transforming the automotive sector into a cleaner, greener, and more sustainable industry. The path ahead may be challenging, but the promise of hydrogen fuel cell technology is too significant to ignore in our journey toward a cleaner and more sustainable transportation ecosystem.
If you’d like to dive deeper into this subject, there’s more to discover on this page: Hydrogen fuel cell cars: what you need to know | BMW.com
More links
Additionally, you can find further information on this topic by visiting this page: Hydrogen Fuel Cell Electric Cars | DriveClean